Abstract
Background:Hemophilia A (HA) is an X-linked monogenic coagulation disorder resulting from deficiency of the factor VIII (FVIII, F8) gene in the intrinsic coagulation cascade. The current treatment of HA is based on protein replacement therapy (PRT) through plasma-derived coagulation factors or recombinant proteins with limitations of short half-life, high cost, and life-time requirement of the treatment. Gene therapy has become a promising treatment for HA.
Methods and Materials: We developed an advanced lentiviral vector (LV) system for intravenous (iv) F8 gene therapy. A selective codon optimized and B-domain deleted human F8 (hF8BDD) gene was synthesized, sequenced and functionally verified. LVs carrying a universal EF1α promoter, or several modified tissue-specific promoters including endothelial- (VEC), endothelial and epithelial- (KDR), and two megakaryocyte-specific (ITGA and Gp) promoters, were biologically and immunologically characterized in vitro using human endothelial and megakaryocytic cell lines, EA-hy926 and DAMI, and in vivo using F8 knockout (KO) mice.
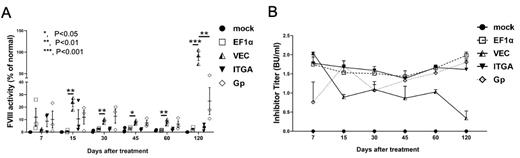
Results: We investigated the different LV promoter constructs and found that LV-VEC-F8BDD exhibited the highest virus packaging (1.3x10 9 transduction units/ml) and transduction efficiencies compared with the other LV constructs. Tissue-specific expression of the VEC, KDR, ITGA and Gp promoters was confirmed in EA-hy926 and DAMI cells by RT-PCR, Western blot and ELISA analyses. We detected F8 activities close to 6 folds and 4.5 folds above the normal plasma level from the EF1a LV-F8BDD transduced EA-hy926 cells and DAMI cells, respectively, whereas the VEC LV in EA-hy926 cells and the ITGA-LV in DAMI cells exhibited F8 activities at 1.5 folds and 5 folds above the normal plasma level, respectively. In vivo studies in F8 KO mice via iv injection of LVs after reduced radiation conditioning illustrated preferential vector expression in different cell lineages, with high expression of EF1a vector in CD11b, F4/80 and Ly-6G positive immune cells, and preferential expression of VEC vector in CD31 positive endothelial cells, and ITGA and Gp vectors in CD41 positive megakaryocytes. In addition, we detected variable phenotypic corrections as well as anti-F8 immune responses in the F8 KO mice treated with the different LVs. The iv deliveries of VEC and Gp F8BDD vectors illustrated therapeutic F8 activities over time, around 25% and 8%, respectively, in 60 days, which increased to high levels (80% and 25%, respectively) after 120 days (Figure A). Kinetic analyses of anti-F8 IgG and inhibitor titers (Bethesda assay) of the treated mice showed that the VEC vector exhibited the lowest F8 inhibitory immune response over time (Figure B).
Conclusion: Based on the in vitro and in vivo studies, our results suggest that for HA gene therapy, optimal rather than high F8 expression is critical, and tissue-specific expression but not universal expression can reduce adverse inhibitor effect. We demonstrated that the LV-VEC-F8BDD vector displayed high tissue specificity in vivo, and high transgene delivery efficiency, high coagulation function and low immunogenicity. In addition, iv LV gene therapy could be a safe, convenient and effective HA gene therapy strategy.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal